Details of the Drug
General Information of Drug (ID: DMGBKVD)
| Drug Name |
Gamma Hydroxybutyric Acid
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Anetamin; GHB; Hydroxyb; Oxybate; SHB; Somsanit; Xyrem; Gamma OH; Oxybate sodium; Utyric acid monosodium salt; EB 27; WY 3478; Gam-OH; Gamma-Hydroxy butyrate; Gamma-Hydroxy sodium butyrate; Gamma-Hydroxybutanoic acid; Gamma-Hydroxybutyrate sodium; Gamma-Hydroxybutyric acid; Gamma-Hydroxybutyric acid decomposition product; Gamma-Hydroxybutyric acid monosodium salt; Gamma-OH; Xyrem (TN); Gamma-Hydroxybutyric acid, sodium salt; Oxy-n-butyric acid; Sodium Oxybate (4-hydroxybutanoic acid); Sodium .gamma.-hydroxybutyrate; Sodium .gamma.-oxybutyrate; AA3E2AF0-AB7A-4A1E-A391-199C049D7162; Butanoic acid, 4-hydroxy-, monosodium salt; Butanoic acid, 4-hydroxy-, sodium salt; Butyric acid, 4-hydroxy-, monosodium salt; Butyric acid, 4-hydroxy-, sodium salt; 3-carboxypropoxy acid; 4 HB; 4-Hydroxy; 4-Hydroxyacid; 4-Hydroxyalkanoic acid; 4-Hydroxybutanoic acid; 4-Hydroxybuttersaeure; 4-Hydroxybutyrate sodium; 4-Hydroxybutyric acid; 4-Hydroxybutyric acid monosodium salt; 4-Hydroxycarboxylic acid; 4-OHB; 4-hydroxy-butyric acid
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Anesthetics
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
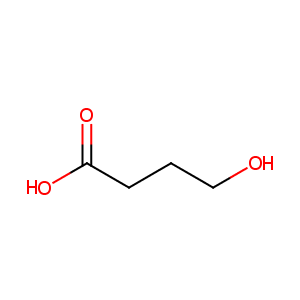 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 104.1 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.6 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
